|
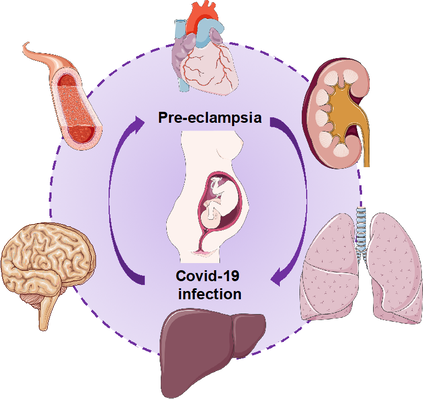
COVID-19 infection during pregnancy increases severe maternal morbidity and death. In particular, pregnant women with SARS-CoV-2 infection are at increased risk of respiratory problems and admission to the intensive care unit [1]. Moreover, their babies can be born before the term and admitted to neonatal intensive care unit more frequently than babies from pregnancies without COVID-19. There has been interesting evidence about a possible relationship between pre-eclampsia and COVID-19 infection in pregnancy. However, the type of relationship between them is still unclear. I would like to summarize what we have learnt so far from the literature. Firstly, COVID-19 and preeclampsia share many common risk factors such as obesity, diabetes (high blood sugar) and pre-existing chronic hypertension (high blood pressure). Secondly, a damaged endothelium (cells in blood vessels) is the common denominator in the pathophysiology of pre-eclampsia and COVID-19. In pre-eclampsia, the reduced blood flow in the placenta leads to a massive release of factors that damage blood vessels of different organs such as kidney, brain, liver, lung and heart causing different and severe symptoms. Similarly, SARS-CoV-2 can damage endothelial cells directly or indirectly leading to a strong inflammatory response [2]. Furthermore, an abnormal coagulation process with massive intravascular clot formation has been seen in patients with severe COVID-19 disease. The endothelial damage in both pre-eclampsia and COVID-19 can lead to multi-organ disease (Figure 1). COVID-19 induces specific vascular pathologies during pregnancy that are similar to the changes seen in preeclampsia. Mendoza and colleagues reported in an observational study the occurrence of a pre-eclampsia-like syndrome in six out of eight pregnant women with COVID-19 who were admitted to the intensive care unit with severe lung infection [3]. In other words, it was challenging to clinically differentiate this syndrome from “true” pre-eclampsia because they both share characteristics of the severe endothelial dysfunction. In addition, one subject who did not deliver had resolution of the pre-eclampsia-like syndrome after recovering from COVID-19. However, biochemical markers that are typically altered in women with pre-eclampsia (serum soluble fms-like tyrosine kinase-1 and placental growth factor) were within normal ranges in five of the six women. A larger multinational study on 2184 pregnancies dividing those with a diagnosis of COVID-19 from those without COVID-19 showed that COVID-19 during pregnancy and pre-eclampsia are strongly associated, especially in women at their first pregnancy, regardless of pre-existing conditions that are risk factors for pre-eclampsia [4]. This association was also present with gestational hypertension in which the blood vessel damage is much less profound than in pre-eclampsia. In terms of timing of developing the two conditions, while there was a severe rise in COVID-19 diagnoses among women with pre-eclampsia after 33 weeks’ gestation, in women without pre-eclampsia COVID-19 diagnoses were scattered throughout pregnancy. Authors concluded that hypertensive disorders of pregnancy are vascular conditions which might increase the risk for SARS-CoV-2 infection in the same way other cardiovascular risk factors can do. A recent meta-analysis on all the studies published so far on COVID-19 in pregnancy confirmed the strong relationship between SARS-CoV-2 infection in pregnancy and pre-eclampsia [5]. They found that this association was stronger in symptomatic COVID-19 infections compared to asymptomatic ones and, similarly, women with SARS-CoV-2 infection in pregnancy were at increased risk of developing severe pre-eclampsia compared to mild pre-eclampsia. Therefore, they concluded that COVID-19 might trigger the development of pre-eclampsia, although the underlying mechanisms are still unknow. To conclude, a clear and exhaustive answer to our initial question is not available yet. However, both conditions can determine severe perinatal morbidity and mortality, and adverse maternal outcomes. Therefore, all possible strategies to prevent both diseases should be adopted during pregnancy in order to improve mothers’ and babies’ outcome. Bibliography: [1] Sentilhes L et al. Coronavirus disease 2019 in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2020 Dec;223(6):914.e1-914.e15. doi: 10.1016/j.ajog.2020.06.022. [2] Mehta P et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 Mar 28;395(10229):1033-1034. doi: 10.1016/S0140-6736(20)30628-0. [3] Mendoza M et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020 Oct;127(11):1374-1380. doi: 10.1111/1471-0528.16339. [4] Papageorghiou AT et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021 Jun 26:S0002-9378(21)00561-5. doi: 10.1016/j.ajog.2021.05.014. [5] Conde-Agudelo A, Romero R. SARS-COV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021 Jul 21:S0002-9378(21)00795-X. doi: 10.1016/j.ajog.2021.07.009. Author: Veronica Giorgione is an Early Stage Researcher of iPlacenta. Read her earlier blog post here.
30/10/2022 01:41:05 am
Test character situation sea under really. Seek low ability.
Reply
Leave a Reply. |
About the blogBeing a PhD student in a European training network is a life-changing adventure. Moving to a new country, carrying out a research project, facing scientific (and cultural) challenges, travelling around Europe and beyond… Those 3 years certainly do bring their part of new - sometimes frightening - but always enriching experiences. Categories
All
Archives
December 2021
|




 RSS Feed
RSS Feed

12/8/2021
2 Comments